By mid-2025, the pharmaceutical industry will face its biggest shake-up in decades. Dozens of top-selling drugs-some bringing in billions each year-will lose patent protection. This isn’t just a legal detail. It’s a turning point for patients, pharmacies, hospitals, and drugmakers. The blockbuster patent expirations starting in 2025 will slash prices, open the door to generics, and force companies to rethink how they make money. If you’ve ever paid hundreds of dollars a month for a prescription, this directly affects you.
What’s Actually Expanding in 2025?
The first major hit comes in July 2025: Novartis’ Entresto (sacubitril/valsartan). This heart failure drug brought in $7.8 billion globally in 2024. Its core patent expires that month, and generic versions are already approved. One patient in California told her doctor she’s been paying $300 a month for Entresto. With generics, that could drop to under $40. That’s an 85% price drop overnight.
Entresto isn’t alone. The FDA has already approved the first generic version of sacubitril-its key component-months before the patent even expires. That’s unusual. It shows how much pressure is building. Generic manufacturers have been preparing for years. Over 127 applications for drugs expiring in 2025 were filed last year alone, up 27% from the year before.
The Big Three: Keytruda, Eliquis, and Ibrance
After Entresto, the next wave hits hard:
- Keytruda (pembrolizumab) from Merck: This cancer immunotherapy made $29.3 billion in 2024. It’s the best-selling drug in the world right now. Its main patent expires in 2028. Experts predict Merck could lose $15 billion in annual revenue within 18 months of generic entry. No other drug comes close to that kind of financial cliff.
- Eliquis (apixaban) from Bristol Myers Squibb and Pfizer: This blood thinner brought in $13.2 billion in 2024. Its key patent expires in November 2026. With multiple generics already in the pipeline, prices will likely crash fast. Patients already pay $100-$200 a month. Generics could bring that down to $10-$20.
- Ibrance (palbociclib) from Pfizer: A breast cancer drug with $5.4 billion in 2024 sales. Its patent expires in 2026. Like Eliquis, it’s in a crowded space. Once generics arrive, market share will shift quickly.
These three alone account for over $47 billion in annual sales at risk. And they’re not outliers-they’re the tip of the iceberg.
Why This Wave Is Different
Patents have expired before. But never like this. In the past, one or two big drugs would lose protection each year. Now, 65 drugs with over $100 million in annual sales each are set to expire between 2025 and 2030. That’s $187 billion in global revenue up for grabs.
Two things make this unique:
- Scale: The total value at risk is bigger than the entire pharmaceutical market of many countries.
- Complexity: Not all drugs are the same. Small-molecule pills like Eliquis are easy to copy. Biologics like Keytruda are made from living cells. Copying them is harder. That’s why biosimilars take longer to launch-18 to 24 months versus 6 to 12 for regular generics.
And it’s not just about patents. Companies stack them. A single drug like Keytruda has over 130 patents covering everything from its chemical structure to how it’s packaged. That’s why some generics are still blocked-even after the main patent expires. But courts are starting to roll back these tactics. The FTC is investigating more "pay-for-delay" deals than ever.

Who Wins? Who Loses?
The winners are clear: patients and the healthcare system.
By 2030, the Congressional Budget Office estimates these expirations will cut U.S. healthcare spending by $312 billion over the decade. That’s $198 billion saved just between 2025 and 2027. For heart failure patients, that could mean $3,000 a year in out-of-pocket savings. For cancer patients on Keytruda, it could mean thousands less per year in co-pays.
Generic drugmakers are also winning. Teva, Mylan, and Sandoz are racing to be first to market. Teva alone has 37 products in development targeting these expirations. The first generic to enter usually captures 45% of the market in the first month. By year one, it’s over 90% for pills like Entresto and Eliquis.
The losers? The big drugmakers. Merck could lose 41% of its blockbuster revenue by 2030. Amgen could lose over half of its current revenue. That’s why Merck just announced a $12 billion investment in next-gen cancer drugs. Bristol Myers Squibb bought Karuna Therapeutics for $4.1 billion to build a new pipeline. They’re not waiting for the storm-they’re trying to build a new ship.
What’s Happening Behind the Scenes
It’s not just about the drugs. Hospitals, pharmacies, and insurers are preparing too.
According to the American Society of Health-System Pharmacists, 87% of hospital pharmacy directors are already changing their formularies-meaning they’re swapping out brand-name drugs for generics before the patent even expires. Some are already negotiating 60% price cuts with insurers.
Pharmacists are training staff. Patients are being warned. On Reddit, pharmacists are sharing how they’re preparing: "We’re updating our computer systems to auto-substitute generics. We’re printing new patient handouts. We’re calling patients before their refill date."
But there are risks. When Humira’s biosimilars launched, there were supply shortages. Some patients waited weeks. Pharmacies on Facebook are already worried the same thing will happen with Entresto. Manufacturers are ramping up-but can they keep up?

How to Prepare: What Patients and Providers Should Know
If you’re on one of these drugs, here’s what to do:
- Know your drug: Is it a small molecule (pill) or a biologic (injection)? Small molecules become generic faster.
- Ask your doctor: "Will there be a generic soon? When?" Don’t assume they know-many aren’t tracking patent dates.
- Check your pharmacy: Call ahead. Ask if they have the generic. Some won’t stock it until after the patent expires, even if it’s approved.
- Review your insurance: Many plans will automatically switch you to the cheaper version. But not all. Ask if you need to request it.
For providers: Start talking to patients now. Don’t wait until the last minute. Use tools like the FDA’s Orange Book (for pills) and Purple Book (for biologics) to track expirations. The Generic Pharmaceutical Association also offers a free "Patent Cliff Navigator" tool used by 82% of pharmacy benefit managers.
What Comes After 2030?
The 2025-2030 wave is massive-but it’s not the end. Another wave is already building. Drugs like J&J’s Stelara and AbbVie’s Rinvoq are set to expire in the early 2030s. Meanwhile, new therapies in gene editing and targeted cancer drugs are emerging. But they’re expensive. The real question isn’t just how much we’ll save-it’s whether the next generation of drugs will be affordable at all.
For now, the focus is on these next five years. The money saved will help millions afford care. The companies that adapt will survive. The ones that don’t? They’ll fade into the background, just like the old blockbuster drugs they once dominated.
Which blockbuster drugs lose patent protection in 2025?
The most significant drug losing patent protection in 2025 is Novartis’ Entresto (sacubitril/valsartan), a heart failure medication with $7.8 billion in 2024 sales. Its core U.S. patent expires in July 2025. Generic versions are already approved by the FDA, with multiple manufacturers ready to launch immediately. Other drugs like Humira biosimilars have already entered the market, but Entresto is the largest single-value expiration this year.
When does Keytruda’s patent expire?
Merck’s Keytruda (pembrolizumab), the world’s top-selling drug with $29.3 billion in 2024 revenue, will lose its core composition-of-matter patent in 2028. This is the most financially significant patent expiration in pharmaceutical history. Biosimilars are expected to enter the market 18-24 months after the patent expires, with generic competition likely to reduce prices by 70-80% within two years.
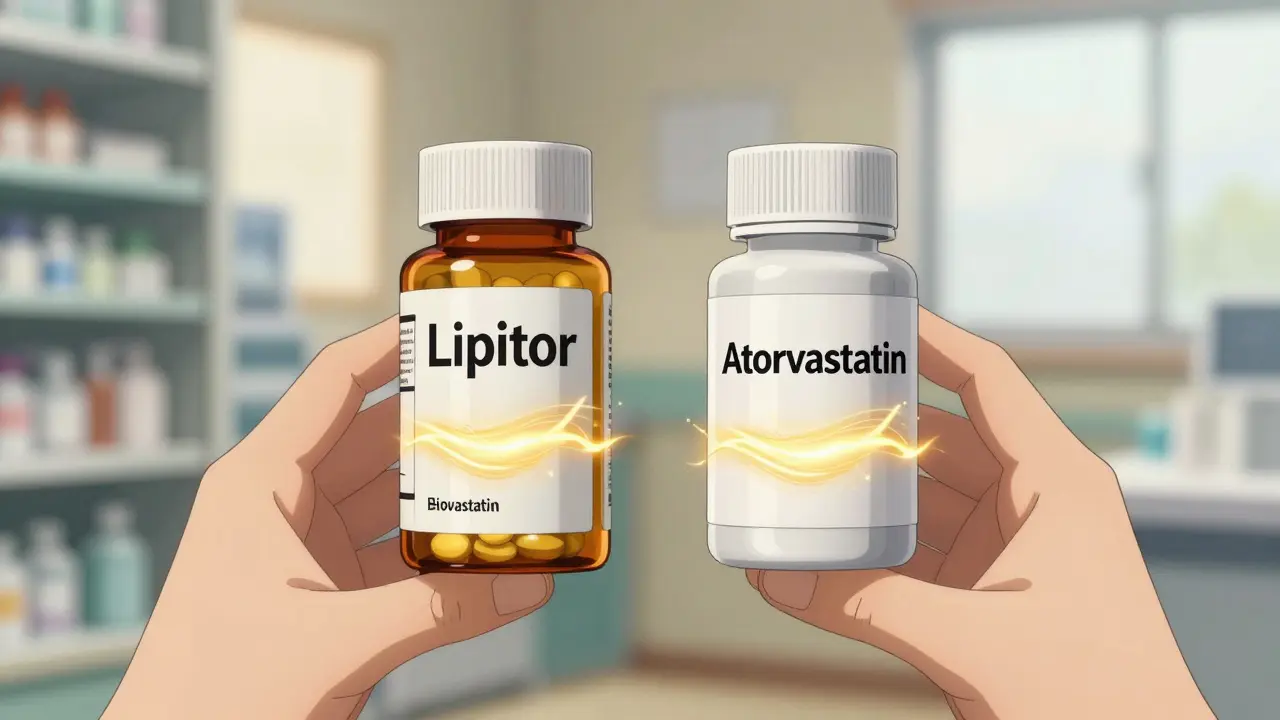
How much will generic Entresto cost compared to the brand?
Patients currently pay $150-$300 per month for brand-name Entresto. Generic versions are expected to cost $20-$40 per month-a drop of 85% or more. Many insurers will automatically switch patients to the generic upon launch. Some pharmacy benefit managers have already negotiated 60% price reductions in anticipation of the July 2025 expiration.
Why are biologics like Keytruda harder to copy than pills like Eliquis?
Biologics like Keytruda are made from living cells, making them structurally complex. Their exact structure can’t be perfectly replicated, so copies are called biosimilars, not generics. Developing a biosimilar takes 3-4 years and costs over $100 million. Small-molecule drugs like Eliquis are chemical compounds that can be copied exactly, so generics are cheaper and faster to produce-typically under $2.6 million and 3 years.
Will my insurance automatically switch me to a generic?
Most insurers will automatically switch you to a generic or biosimilar once it’s available, especially for high-cost drugs like Entresto and Eliquis. But you should confirm with your plan. Some require prior authorization or a formulary exception. Always check with your pharmacy or insurer before your next refill to avoid delays or unexpected costs.
What’s the difference between a generic and a biosimilar?
Generics are exact copies of small-molecule drugs, like pills (e.g., Eliquis). Biosimilars are highly similar-but not identical-to complex biologic drugs made from living cells (e.g., Keytruda). Biosimilars require more testing and take longer to approve. They also typically cost less than the brand but not as much as generics. Price reductions for biosimilars average 30-40% initially, compared to 80-90% for generics.
How will patent expirations affect drug availability?
Availability should improve over time as multiple manufacturers enter the market. But initial shortages are possible, as seen with Humira biosimilars. Supply chains are still scaling up. Pharmacies and hospitals are preparing by ordering extra stock ahead of expiration dates. If your medication is in short supply, ask your pharmacist for alternatives or a temporary refill.
What should I do if my drug is about to go generic?
Talk to your doctor about switching. Ask your pharmacist when the generic will be available. Check with your insurance to confirm coverage. Don’t stop taking your medication. Most generics are just as safe and effective. The switch can save you hundreds or even thousands per year. Many patients report no difference in how they feel after switching.



Charles Barry
December 23, 2025 AT 16:47This is all a orchestrated scam by Big Pharma and the FDA to flush out cash before the real drugs arrive. Entresto? That’s just a placebo with fancy packaging. The real active ingredient was patented in 1997 by a Chinese lab and stolen. You think generics are cheap now? Wait till the black market starts shipping unregulated versions from India-$5 a bottle, no prescription needed. And don’t get me started on the AI-driven price-fixing algorithms the insurers are using to jack up co-pays right before generics drop. They’re milking you till the last second.
They’re lying about the savings. The real cost? Your data. Your health records. Your DNA. They’re harvesting it all through the "generic switch" forms. You think this is about affordability? It’s about control.
Mark my words-by 2027, your pill bottle will have a QR code that logs your heartbeat. And you’ll be grateful for it.
suhani mathur
December 24, 2025 AT 07:42Oh honey, you’re telling me people are gonna pay $40 instead of $300 for Entresto? Sweet. Now tell me why my cousin in Mumbai still pays $120 for a 30-day supply of generic metformin because the local pharmacy "ran out"-again.
Yes, the math looks great on paper. But in real life? Supply chains collapse, middlemen vanish, and pharmacists get paid in promises. I’ve seen this movie before. The savings? They’ll be real for the rich. For the rest of us? We’ll be waiting in line with a note from our doctor and a prayer.
But hey-at least the big pharma CEOs will finally have to wear the same socks twice. Progress.
Adarsh Dubey
December 24, 2025 AT 11:00The data presented here is largely accurate and well-sourced. The distinction between small-molecule generics and biosimilars is particularly well-articulated. The projected $187 billion in revenue loss between 2025 and 2030 aligns with peer-reviewed analyses from Health Affairs and the Congressional Budget Office.
However, one underreported factor is the impact on R&D investment. While blockbuster revenue declines, many firms are redirecting capital toward precision oncology and RNA-based therapeutics, which may yield more sustainable long-term returns. The transition is painful, but not unprecedented-recall the statin wave of the early 2000s.
Patients should indeed verify formulary changes with their insurers, but the broader systemic shift toward value-based pricing is inevitable and largely positive. The key is ensuring equitable access, not just lower prices.
Bartholomew Henry Allen
December 26, 2025 AT 01:50The U.S. pharmaceutical industry is the most innovative in the world. We fund 70 percent of global R&D. Letting generics flood the market is economic suicide. We built this. We deserve to profit. No other country has the infrastructure or the will to develop these drugs. Why should we subsidize the rest of the world?
Patents are not loopholes. They are the reward for decades of risk. If you want cheap medicine go to China. Or India. Or Russia. But dont blame us for being the ones who made it possible.
Stop whining. Start investing. Or get off the innovation train.
Wilton Holliday
December 26, 2025 AT 02:50Big win for patients!!! 💪
Imagine telling your grandma she can now afford her heart meds without choosing between groceries and pills. That’s real change.
Shoutout to the generic makers who’ve been grinding for years to get these approved. And to the pharmacists who are updating systems and calling patients-y’all are heroes.
If you’re on one of these drugs, don’t panic. Talk to your doc. Ask your pharmacist. You’ve got this. And you’re not alone. We’re all in this together. 💙
Let’s make sure the savings actually reach people. No middlemen. No delays. Just medicine. 🙌
Raja P
December 27, 2025 AT 22:29Man I remember when I had to pay $400 a month for my dad’s Eliquis before the generics came in. We were barely surviving. Then one day the pharmacy just handed me a little white bottle for $18 and said "you’re good now".
It felt like winning the lottery but with more paperwork.
So yeah, this is happening. And it’s gonna save lives. Not just money. Lives.
Let’s hope the big boys don’t screw it up with supply issues like they did with Humira. I’ve seen people go without for weeks. That’s not okay.
Joseph Manuel
December 29, 2025 AT 11:04The assertion that patent expirations will reduce healthcare expenditures by $312 billion over a decade is mathematically plausible but empirically unsupported by longitudinal data. The CBO projections assume perfect substitution, zero adverse events, and linear price elasticity-all of which are violated in real-world markets.
Furthermore, the transition to biosimilars introduces new administrative burdens, increased monitoring costs, and potential liability exposure. The net fiscal impact remains uncertain.
While patient savings are desirable, policy must account for systemic complexity, not merely headline figures.
Harsh Khandelwal
December 30, 2025 AT 14:06So they’re gonna drop the price of Entresto to $40? LMAO. That’s the same price as a bag of chips in a hospital vending machine. You think the pharma bros are just gonna roll over? Nah. They’re already selling "premium" versions with fancy packaging and Bluetooth trackers. "This one glows when you take it!"
And don’t even get me started on the "generic" Entresto that’s actually just sugar pills with a label that says "FDA approved". Been there. Done that. Got the t-shirt. And the kidney stones.
Next thing you know, they’ll patent the air you breathe. Again.
They always win. We just get to pick the color of our chains.
Andy Grace
December 30, 2025 AT 19:12I’ve worked in community pharmacy for 18 years. I’ve seen patients cry because they can’t afford their meds. I’ve seen them skip doses. I’ve seen them die because the system didn’t care.
This? This is the first time in my career I’ve seen real momentum toward change. Not just talk. Not just policy papers. Real, tangible, life-saving access.
Yes, it’s messy. Yes, there will be hiccups. But for the first time, the people who need these drugs are being heard.
Let’s not ruin it by overcomplicating it. Just let them have their medicine.