Medication Reformulation Impact Calculator
How Reformulation Improves Your Medication
Enter your current medication details below to see potential benefits of reformulation. Reformulations maintain the same active ingredient while improving how the drug works for you.
Potential Benefits
Dose Reduction
You might need fewer doses per day.
Quality of Life
This reformulation could improve your quality of life by .
Why this matters
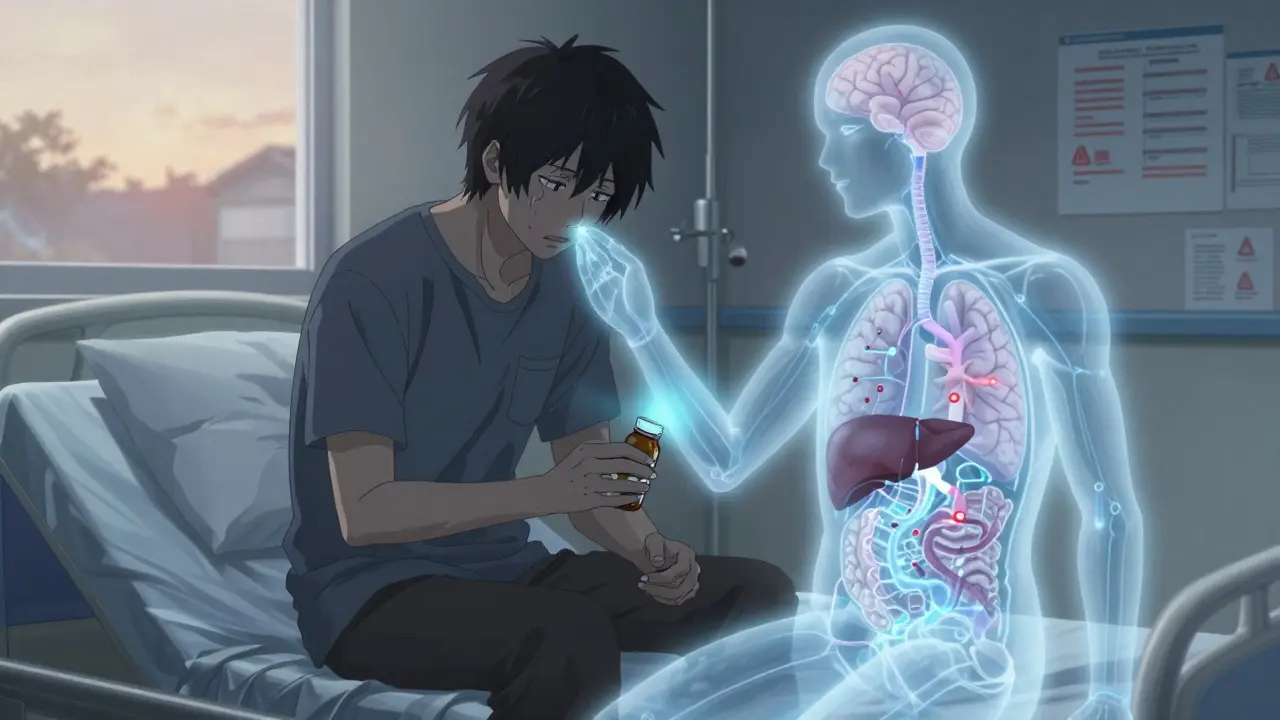
Have you ever picked up your prescription and noticed the pill looks different? Maybe it’s a different color, shape, or even a liquid instead of a tablet? That’s not a mistake. It’s medication reformulation-a quiet but powerful shift happening behind the scenes in pharmacies and labs across the world.
What Exactly Is Medication Reformulation?
Medication reformulation means changing how a drug is made-without changing the active ingredient. Think of it like upgrading a car engine without swapping out the fuel. The same medicine is still working inside your body, but now it’s delivered differently, lasts longer, or is easier to take.
The active pharmaceutical ingredient (API) stays the same. But everything else? That’s fair game. Excipients-those inactive ingredients like binders, coatings, or flavorings-can be swapped. The release pattern can be tweaked so the drug hits your bloodstream slower or steadier. Even the route of administration can change: a pill becomes a patch, an injection becomes an inhaler, or a daily tablet turns into a once-weekly capsule.
This isn’t new. The FDA created the 505(b)(2) pathway back in the 1980s to make it easier for companies to approve these kinds of changes. Instead of starting from scratch with years of clinical trials, they can use existing safety data from the original drug. That cuts development time and cost dramatically.
Why Do Companies Do This?
There’s more than profit at stake here. While extending patent life is one reason-sometimes called “evergreening”-many reformulations are driven by real patient needs.
Take orphan drugs, for example. These are treatments for rare diseases. Often, the original version requires painful injections or must be taken multiple times a day. A reformulation might turn that into a simple oral tablet. Suddenly, a child with a rare condition can take their medicine at school instead of visiting a clinic every morning. Compliance skyrockets. Quality of life improves.
Another big driver? Side effects. Some people can’t tolerate the original formulation because of a specific excipient-maybe a dye, gluten, or lactose. Reformulating removes that trigger. A 2022 case study from Kymanox showed a mid-sized company successfully reformulated an orphan drug in just 12 months, improving patient adherence and reducing nausea by switching to a slower-release coating.
And then there’s bioavailability. Sometimes, the original drug doesn’t get absorbed well. Reformulation can fix that. By tweaking particle size, adding solubilizers, or using new polymers, companies can make sure more of the medicine actually enters the bloodstream. That means lower doses, fewer side effects, and better outcomes.
How Is It Different From a New Drug?
Creating a brand-new drug is like building a house from the ground up. It costs around $2.6 billion and takes 10 to 15 years. Success rates? About 10%.
Reformulation? It’s like remodeling. You keep the foundation, but update the plumbing, insulation, and windows. Costs drop to $50-100 million. Timeline? 3 to 5 years. Approval success rate? Around 30%-three times higher.
That’s why big pharma and smaller biotech firms alike are leaning into reformulation. In fact, about 27% of all new drug applications submitted to the FDA each year are reformulations. And globally, reformulated drugs generate roughly $60 billion in annual sales-15% of the prescription drug market.
It’s not just about money. It’s about efficiency. When a drug has already been used by millions, doctors and patients know how it works. Reformulators build on that knowledge. They don’t guess-they improve.
What Changes Are Common in Reformulations?
Not all reformulations are the same. Here are the most frequent types you’ll see:
- Modified release: Extended-release tablets that last 12 or 24 hours instead of 4-6. Think of it like switching from sipping coffee all morning to drinking one large, slow-dissolving brew.
- Route change: Oral to transdermal (patch), injectable to nasal spray, or even intravenous to oral capsule. ZIM Labs reported in 2023 that switching from injections to oral forms in chronic conditions improved adherence by up to 40%.
- Excipient swap: Removing allergens like lactose or dyes, or adding flavoring for children. This is especially common in pediatric and geriatric formulations.
- Dose adjustment: Combining two doses into one pill, or changing strength to match clinical guidelines. A 10mg tablet might become a 5mg tablet with a different coating for better absorption.
- Form factor: Turning a tablet into a chewable, a liquid into a dissolvable film, or a capsule into a sprinkle that can be mixed with food.
Important note: If the active ingredient’s chemical structure changes-like turning a drug into a prodrug-it’s no longer a reformulation. That’s a new molecular entity and requires full approval.
Regulatory Hurdles and Bioequivalence
Even though reformulations use existing data, they still need to prove they work the same way. That’s where bioequivalence comes in.
Regulators require that the reformulated version delivers the same amount of medicine into the bloodstream at the same rate as the original. That’s tested in small clinical studies called bioequivalence trials. If the drug is absorbed differently-say, a patch vs. a pill-then more data is needed. In some cases, full clinical trials are required if the reformulation changes how the drug affects the body.
The FDA’s 2022 guidance made this easier for certain types of reformulations, especially those changing dosage forms for already-approved drugs. Companies now have clearer rules on what data to submit, reducing delays.
But here’s the catch: if the reformulation doesn’t offer a meaningful benefit, regulators can reject it. The goal isn’t just to extend patents-it’s to improve patient care.
Patient Experience: What People Notice
Most patients don’t know they’re taking a reformulated version. But they notice the difference.
“I used to vomit after taking my old pill,” one patient shared in a 2023 survey. “The new version doesn’t upset my stomach. I actually take it now.”
People report:
- Less nausea or dizziness
- Fewer daily doses
- No more injections
- Easier to swallow or taste better
But it’s not perfect. Sometimes, a reformulation introduces new side effects. A coating that works well for adults might break down too fast in kids. A flavor added for children might trigger allergies. Or, in rare cases, a change in excipients reduces absorption, making the drug less effective.
That’s why pharmacists are trained to flag changes. If your medication looks different, ask: “Is this the same drug?” It’s not about suspicion-it’s about safety.
The Future of Reformulation
Reformulation is only getting smarter. New technologies are opening doors:
- 3D-printed pills that can release multiple drugs at different times
- Nanoparticle delivery that targets specific organs, reducing side effects
- Smart patches that release medicine based on body temperature or glucose levels
Orphan drugs are a major focus. With few treatment options, even small improvements-like making a drug taste better or easier to store-can be life-changing.
Industry analysts predict reformulation will keep growing. As drug development for entirely new molecules gets harder and more expensive, improving what already works makes more sense than ever.
What Should You Do If Your Medication Changes?
If your pill changes color, shape, or how often you take it:
- Don’t panic. It’s likely a reformulation, not a mistake.
- Check the label. The active ingredient should be identical.
- Ask your pharmacist: “Is this the same medicine, just changed?”
- Watch for side effects. Report anything new to your doctor.
- Keep taking it unless told otherwise. Most reformulations are safer or more convenient.
Reformulation isn’t about tricking patients. It’s about listening to them. When millions of people use a drug, we learn what works-and what doesn’t. Reformulation turns that real-world feedback into better medicine.
Are reformulated drugs less effective than the original?
No, not if they’ve been properly approved. Regulators require reformulated drugs to prove they deliver the same amount of active ingredient into the bloodstream at the same rate as the original. This is called bioequivalence. If a reformulation changes how the drug works-like making it slower-acting-it must still be proven safe and effective in clinical studies. Most reformulations are just as effective, sometimes even more so because they improve absorption or reduce side effects.
Why does my pill look different now?
Pharmaceutical companies often reformulate drugs to improve patient compliance, reduce side effects, or extend patent life. A change in color, shape, or size usually means the excipients (inactive ingredients) or release mechanism was updated. It doesn’t mean the medicine inside is different. Always check the active ingredient name on the label-it should match your previous version. If you’re unsure, ask your pharmacist.
Can reformulation cause new side effects?
Yes, but it’s rare. Changing the coating, flavoring, or delivery method can sometimes introduce new reactions. For example, a new dye might trigger an allergy, or a slower-release coating might cause bloating in some people. Most reformulations are designed to reduce side effects, not cause them. If you notice something new after switching-like dizziness, rash, or nausea-tell your doctor. It could be related to the reformulation.
Is reformulation just a way for companies to make more money?
Sometimes, yes. Extending patent life by making minor changes is called “evergreening,” and it’s a controversial practice. But many reformulations are driven by real patient needs-like making a pill easier to swallow for elderly patients, removing allergens, or switching from daily injections to weekly patches. The FDA reviews each reformulation for clinical benefit, not just profit. While some changes are small, others have significantly improved quality of life for millions.
How long does it take to reformulate a drug?
It typically takes 12 to 24 months, depending on complexity. Simple changes-like switching excipients or adjusting tablet size-can be done in under a year. More complex changes, like turning an injectable into an oral form, may take up to two years. A 2022 case study showed a mid-sized company completed a full reformulation of an orphan drug in just 12 months by using integrated regulatory and formulation strategies.
Final Thoughts
Medication reformulation isn’t flashy. It doesn’t make headlines like a cure for cancer. But it’s one of the quietest, most effective ways medicine gets better. It takes what we already know works and makes it easier, safer, and more reliable for the people who need it every day.
Next time your pill looks different, don’t assume the worst. Ask questions. Understand the change. And know that behind that small shift in shape or color, there’s a lot of science-and a lot of care-trying to make your treatment work better.



Randolph Rickman
December 17, 2025 AT 00:01I've been on the same med for 8 years and the pill changed last month from blue to white. At first I thought I got the wrong prescription, but my pharmacist explained it was a reformulation. Now I take it once a day instead of three times. No more midday nausea either. This is how medicine should evolve.
Stop panicking when the pill looks different. The active ingredient hasn't changed. Ask your pharmacist. They know.
sue spark
December 18, 2025 AT 07:23My mom switched to a reformulated version of her blood pressure med and suddenly she could swallow it without gagging. The old one was like swallowing a rock. The new one dissolves faster. No more choking episodes at breakfast. Small changes matter.
Also no more lactose. She's been dairy sensitive for years and no one ever told her the pill had it.
Joanna Ebizie
December 19, 2025 AT 21:55Oh great so now they're just repackaging old drugs to keep charging us $500 a month. Evergreening is just corporate greed dressed up as 'patient care'.
My insurance won't cover the new version unless I pay $200 extra. Yeah right. Same active ingredient. Same factory. Just a new label.
Elizabeth Bauman
December 21, 2025 AT 00:57These reformulations are a Trojan horse. The FDA lets them slide because they're pressured by Big Pharma lobbyists. Did you know 70% of these 'improvements' are made by companies that also own the original patent?
Meanwhile, real innovation-like mRNA tech-is buried under red tape. We're letting corporations play the system while real breakthroughs get ignored. This isn't progress. It's manipulation.
And don't get me started on how they add artificial dyes to make pills look 'premium'. That's not medicine. That's marketing.
Dylan Smith
December 21, 2025 AT 06:59I work in clinical trials and I've seen reformulations go from idea to approval in 14 months. That's insane speed compared to new drugs. And honestly? Most of them are lifesavers.
We had a kid with a rare epilepsy drug that required 6 injections a day. Parents were crying every time. The reformulation? Once-a-week oral. The kid started going to school. The mom got a job. That's not profit. That's humanity.
Yeah some are sketchy. But the good ones? They're quiet heroes. Don't throw the baby out with the bathwater.
Mike Smith
December 21, 2025 AT 15:11As a healthcare professional, I want to emphasize that reformulation is one of the most underappreciated advancements in modern pharmacology.
It is not a substitute for innovation, nor is it a betrayal of patient trust. It is, in fact, a responsible application of existing knowledge to enhance therapeutic outcomes.
When a patient can transition from an injectable to an oral formulation with equivalent bioavailability, the impact on adherence, autonomy, and quality of life is profound.
Regulatory pathways exist precisely to ensure that these changes are not arbitrary, but evidence-based. The FDA's 505(b)(2) framework is not a loophole-it is a strategic tool for public health.
Patients who question changes are not being paranoid-they are being informed. And that is commendable.
However, the response to such changes should be inquiry, not alarm. Consult your pharmacist. Review the label. Understand the difference between active ingredient and excipient.
Medicine evolves. We must evolve with it-with discernment, not dogma.
Ron Williams
December 22, 2025 AT 19:39My uncle in India takes a reformulated version of his diabetes med. It's cheaper, tastes better, and doesn't cause the stomach cramps the old one did. He's 78 and still walks 3 miles a day.
Reformulation isn't just a US thing. It's global. In places where meds are scarce, even small improvements mean the difference between life and death.
Big pharma's profit motive? Sure, it's there. But so is the human need. Don't let cynicism blind you to the real wins.
Kitty Price
December 22, 2025 AT 20:28Just got my new patch for migraines. No more pills. No more nausea. Just stick it on and chill 😌
Also the old one made me break out. This one? Zero reactions. My skin is happy. My head is quiet. Thank you science.
Aditya Kumar
December 24, 2025 AT 05:07meh
Colleen Bigelow
December 25, 2025 AT 04:54They're slipping in GMO corn starch and glyphosate-laced fillers under the guise of 'improvement'. You think your pill looks different? Wait till you see what's really inside.
Remember when the FDA approved that 'enhanced' antidepressant that turned out to have a neurotoxin? They called it a 'minor excipient tweak'.
It's not just about profit. It's about control. They want you dependent on their version. They want you too scared to ask questions. But I'm not fooled.
Check the batch code. Look up the excipient suppliers. If it's made by a Chinese subsidiary? Run. They're not playing with your health. They're playing Russian roulette with your neurons.
Billy Poling
December 25, 2025 AT 20:48It is my professional opinion, grounded in extensive review of regulatory documentation and pharmacokinetic literature, that the phenomenon of medication reformulation constitutes a paradigm shift in pharmaceutical development strategy, one that, while ostensibly beneficial, may inadvertently undermine the foundational principles of therapeutic consistency and patient trust.
Consider, for instance, the case wherein a once-daily extended-release formulation replaces a thrice-daily immediate-release version: while bioequivalence may be demonstrated in healthy volunteers under controlled conditions, the pharmacodynamic response in elderly patients with polypharmacy or renal impairment may diverge significantly from the original product due to altered gastric transit time, hepatic metabolism, or intestinal permeability.
Moreover, the regulatory burden placed upon prescribers and pharmacists to discern between ostensibly equivalent products-without access to full formulation schematics-is both ethically and practically untenable.
Furthermore, the economic incentive structure, wherein patent extension is achieved through minimal formulation alterations, constitutes a form of regulatory arbitrage that distorts market competition and inflates healthcare expenditures without commensurate clinical benefit.
It is therefore imperative that clinicians advocate for transparent disclosure of excipient composition, mandatory post-marketing surveillance of reformulated products, and the establishment of a national database to track patient-reported adverse events tied to formulation changes.
Until such safeguards are implemented, we are not advancing medicine-we are engineering uncertainty.
Kim Hines
December 27, 2025 AT 10:46My doctor didn't tell me the new version had a different coating. I started having weird heart palpitations. Turned out the new one had a dye I'm allergic to. Took three months to figure it out.
Pharmacists should be required to call patients when a reformulation happens. Not everyone checks the label.