When your doctor talks about switching from a brand-name drug to a cheaper version, you might hear the words generic or biosimilar-but they’re not the same thing. One is a chemical copy, the other is a biological copy. And confusing them could mean missing out on savings-or worse, making a choice that doesn’t fit your treatment plan.
What’s the real difference between generics and biosimilars?
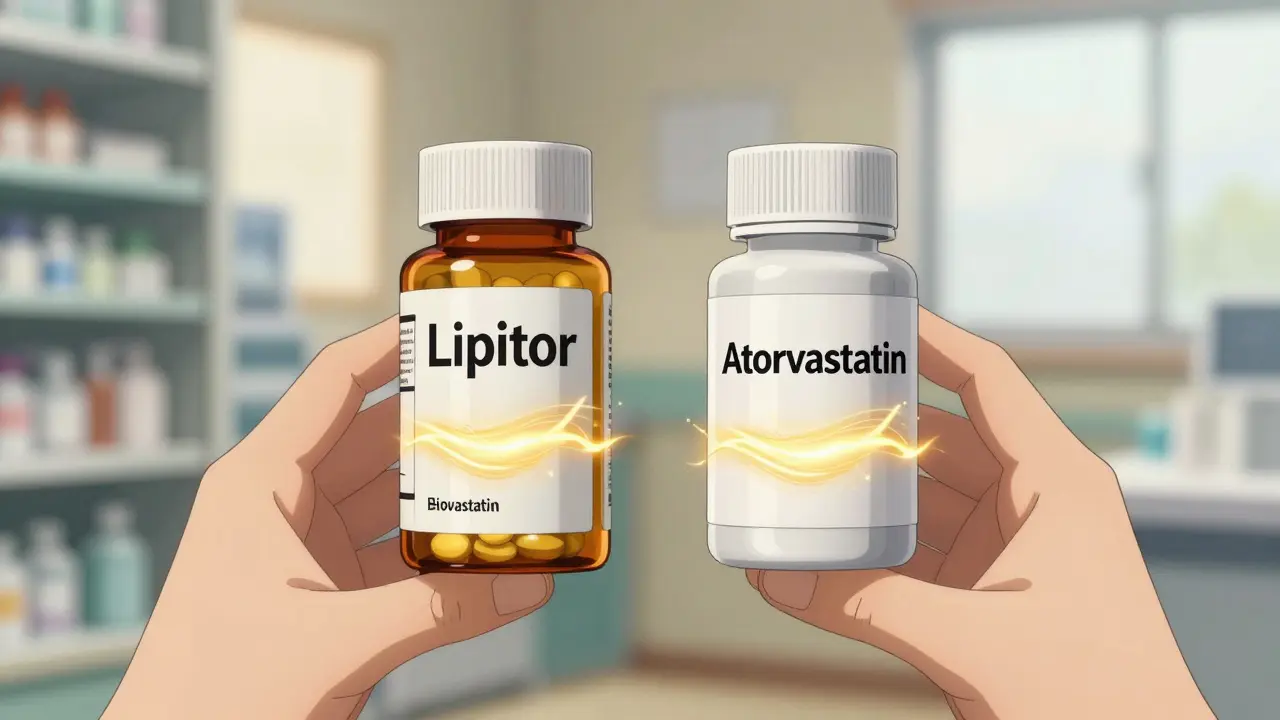
Generics are exact chemical copies of brand-name drugs. Think of them like photocopies of a simple blueprint. If you take a generic version of atorvastatin (the drug in Lipitor), you’re getting the same molecule, in the same dose, with the same effect as the original. The FDA requires generics to match the brand-name drug in strength, dosage, and how your body absorbs it. That’s why millions of people use generics every day for high blood pressure, cholesterol, or thyroid issues-without noticing a difference.
Biosimilars are different. They’re not copies. They’re highly similar versions of complex biologic drugs made from living cells-like proteins, antibodies, or enzymes. These drugs, such as Humira or Herceptin, are used for conditions like rheumatoid arthritis, Crohn’s disease, or certain cancers. Because they’re made by living organisms (not chemically synthesized), even tiny changes in the manufacturing process can affect their structure. That’s why biosimilars aren’t identical to the original-they’re so close that they work the same way in your body, but they can’t be called exact replicas.
Why does this matter for your treatment?
Cost is the biggest reason both exist. Generics cut prices by 80-85% compared to brand-name drugs. A 30-day supply of generic lisinopril might cost $4 instead of $200. Biosimilars don’t save as much-typically 15-20% off the original biologic-but that’s still huge when the original costs $10,000 a year. For someone on a biologic for psoriasis or multiple sclerosis, that 20% could mean the difference between affording treatment or skipping doses.
But here’s the catch: you can’t swap a biosimilar for a generic. They’re not interchangeable. You can’t take a generic version of Humira because it doesn’t exist. Humira is a biologic. Only a biosimilar can replace it. And even then, not all biosimilars are approved for automatic substitution at the pharmacy. Only those labeled “interchangeable” can be switched without your doctor’s permission-and even then, some states require your prescriber to be notified.
How do you know which one you’re getting?
Check the name. Generic drugs usually have the same name as the brand, just without the trademark. For example, metformin is the generic for Glucophage. Biosimilars have longer, more complex names that include a four-letter suffix to tell them apart. For example, the biosimilar for Humira is adalimumab-atto. That suffix is required by the FDA to track safety and side effects.
Look at the form. Generics often come in pills or capsules. Biosimilars are almost always injected or infused-because they’re proteins, your body can’t absorb them orally. You’ll see them as pre-filled pens, syringes, or IV bags. If you’re switching from one biologic to a biosimilar, you might get a different delivery device. Some patients report confusion when the pen looks different, even if the dose is the same.
Are biosimilars safe? What does the data say?
Yes, they’re safe. The FDA approves biosimilars only after reviewing thousands of pages of data: lab tests, animal studies, and clinical trials involving thousands of patients. A 2022 review of 128 studies on biosimilar infliximab (used for Crohn’s and arthritis) found no meaningful difference in safety or effectiveness compared to the original. In fact, the rate of serious side effects was nearly identical.
One concern is immunogenicity-your body reacting to the drug as if it’s foreign. This can cause rashes, fatigue, or even reduce the drug’s effectiveness. But studies show this risk is no higher with biosimilars than with the original biologics. The FDA’s adverse event database shows biosimilar infliximab had 0.12 adverse events per 100 patient-years; the original had 0.15. That’s not a meaningful difference.
Real-world stories back this up. A patient with rheumatoid arthritis in Bristol switched to an adalimumab biosimilar and saved over $8,000 a year-without a flare-up. A colon cancer patient on bevacizumab saw her out-of-pocket cost drop from $450 to $75 per infusion. Her tumor markers stayed stable.
When should you avoid switching?
There are rare cases where switching might not be ideal. If you’re on a drug with a narrow therapeutic index-where even small changes in blood levels can cause harm-your doctor may prefer to stick with the original. But even here, data shows biosimilars are safe. For example, insulin biosimilars like Semglee are now approved as interchangeable, meaning pharmacists can swap them without asking your doctor.
Some patients feel anxious about switching. A 2022 survey found 42% of people with psoriasis worried biosimilars wouldn’t work as well. That’s understandable. But education helps. When your doctor explains the science-how the drug is made, how it’s tested, and why it’s safe-patients feel more confident. Many who were nervous at first end up reporting the same results.
What about cost and insurance?
Generics are almost always covered with low copays. Most insurance plans put them in the lowest tier. Biosimilars are trickier. Some insurers still favor the original biologic because of contracts with manufacturers. Others push biosimilars to save money. Check your formulary. Ask your pharmacist: “Is there a biosimilar for my drug? Is it covered?”
Many biosimilar manufacturers offer patient support programs-like Amgen’s SupportPlus-that help with copay assistance, insurance navigation, and even training on how to use the injection device. These don’t exist for generics, because generics are cheap and simple.
And here’s something new: the Inflation Reduction Act of 2022 removed a financial penalty for doctors who prescribe biosimilars in Medicare Part B. That means more providers are now willing to switch patients, because they’re not losing money on it.

What’s the future?
Biosimilars are growing fast. In 2023, the global market hit $12.4 billion-and it’s expected to reach $58.7 billion by 2030. In Europe, biosimilars make up 65% of the market for drugs they’re approved to replace. In the U.S., it’s only 35%, but that’s climbing. By 2027, nearly half of all biologic prescriptions in the U.S. could be biosimilars.
More approvals are coming. The first interchangeable biosimilar for Stelara (ustekinumab) is expected in 2024. That’s a blockbuster drug with $5 billion in annual sales. If it gets replaced by a biosimilar, patients could save billions.
Meanwhile, generics continue to dominate. Over 11,000 are approved in the U.S. They’re the backbone of affordable care for conditions like diabetes, depression, and infections.
What should you do next?
If you’re on a brand-name drug and wondering if you can switch:
- Ask your doctor: “Is there a generic or biosimilar version of this drug?”
- Check the drug’s name. If it’s a small molecule (pill), it’s likely a generic candidate. If it’s injected and used for autoimmune or cancer conditions, it’s probably a biologic-and a biosimilar may be available.
- Call your pharmacy. Ask if they have a biosimilar or generic on hand, and what your out-of-pocket cost would be.
- Don’t assume switching is risky. The science says it’s safe. The data says it works.
- If you’re nervous, ask for a patient education resource. Many manufacturers offer free videos, brochures, or one-on-one counseling.
There’s no one-size-fits-all answer. But if you’re paying hundreds or thousands a month for a medication, it’s worth asking. You might be eligible for a cheaper, equally effective option.
Are biosimilars as safe as the original biologic drugs?
Yes. The FDA requires biosimilars to undergo extensive testing-analytical studies, animal trials, and clinical trials involving thousands of patients-to prove they have no clinically meaningful differences in safety, purity, or potency. Real-world data from over 38,000 patients shows biosimilars perform just like the original drugs in treating conditions like rheumatoid arthritis and cancer. Adverse event rates are nearly identical.
Can my pharmacist switch my biologic to a biosimilar without asking my doctor?
Only if the biosimilar is labeled “interchangeable” and your state allows it. As of 2024, only a handful of biosimilars have received interchangeable status from the FDA-like Semglee (insulin) and Cyltezo (adalimumab). Even then, 28 states require pharmacists to notify your doctor within 72 hours of substitution. Always check your state’s rules and ask your pharmacist before any switch.
Why are biosimilars so much more expensive to develop than generics?
Generics are made through chemical synthesis-they’re like copying a simple recipe. Biosimilars are made using living cells, which are far more complex. A single batch can vary slightly based on temperature, nutrients, and cell health. To prove they’re safe, manufacturers must run thousands of lab tests, animal studies, and clinical trials. Development costs for a biosimilar average $100-250 million, compared to $2-3 million for a generic.
Do I need to get blood tests more often if I switch to a biosimilar?
No. If your doctor was monitoring your drug levels or disease markers before the switch, they’ll continue doing the same after. There’s no evidence that biosimilars require more frequent testing. The FDA doesn’t require additional monitoring beyond what’s already standard for the reference product.
Why aren’t more doctors prescribing biosimilars?
Many doctors aren’t familiar with the regulatory differences or the latest data. A 2023 survey found only 58% of non-specialist physicians felt very confident prescribing biosimilars. Also, some insurers still favor original biologics due to rebate deals. Patient concerns and complex prior authorization processes also slow adoption. But awareness is growing-especially in oncology and rheumatology, where biosimilars are now standard of care.
Bottom line: Choose based on your drug, not your fear
Generics are for pills. Biosimilars are for injections. One isn’t better than the other-they’re just different tools for different jobs. If you’re on a biologic, a biosimilar could save you thousands. If you’re on a pill, a generic is almost always the smartest choice. Talk to your doctor. Ask your pharmacist. Look up your drug in the FDA’s Purple Book (for biosimilars) or Orange Book (for generics). You don’t have to accept a high price tag if there’s a safe, approved, cheaper option waiting.


James Nicoll
January 27, 2026 AT 16:28So let me get this straight - we’re paying $10k a year for a biologic because Big Pharma says so, but the science says a $2k biosimilar works just as well? Classic capitalism. I’d call it fraud if it weren’t so predictable. 🤡
Joanna Domżalska
January 28, 2026 AT 21:26Wait so you’re telling me a pill and a shot are somehow the same thing? That’s not science, that’s magic. Also, who even decided we should trust proteins made by cells? Sounds like a sci-fi movie.
Faisal Mohamed
January 29, 2026 AT 15:38From a pharmacoeconomic standpoint, the marginal utility curve of biosimilar adoption is non-linear due to regulatory friction and asymmetric information asymmetry in primary care ecosystems. Also, the immunogenicity risk profile is statistically negligible but psychologically salient - which is why we still have this whole debate. 🤓
Josh josh
January 30, 2026 AT 01:58generic = cheap pill biosimilar = expensive shot but still cheaper than the original lol who knew medicine was just capitalism with needles
Conor Flannelly
January 30, 2026 AT 09:11I’ve been on adalimumab for eight years. Switched to the biosimilar last year. My joints didn’t care. My wallet did. I saved nearly $9k. The pen looked different, sure - but so did the coffee machine at my local shop after they changed suppliers. No one asked if the espresso was ‘the same.’ Why do we treat medicine like it’s sacred? The data’s there. The FDA didn’t screw up. And yet, people still whisper like it’s witchcraft. 🤷♂️
It’s not about fear of the new. It’s about fear of being told you were overpaying. And that stings more than any side effect.
I work in a pharmacy in Galway. I’ve seen the same patient come in crying because they couldn’t afford Humira. Then they get the biosimilar. Same dose. Same results. Same dignity. Just cheaper. That’s not science. That’s justice.
And yes, I’ve read the 128 studies. Yes, I’ve seen the adverse event logs. The numbers are practically identical. The only difference is the price tag and the panic.
It’s funny how we’ll trust a $5 generic for blood pressure but get nervous about a $2k biosimilar for arthritis. One’s a molecule. The other’s a protein. But both are made by humans. Both are tested. Both work.
I don’t blame patients. I blame the system that lets a drug cost more than a car. I blame the insurers who still push the original because of rebate deals. I blame the doctors who don’t know the difference between a biosimilar and a placebo.
But mostly? I blame the silence. The silence from those who’ve been saved by these drugs. The silence from those who’ve seen their parents skip doses because of cost. The silence from those who think ‘it’s fine’ because they don’t have to pay.
There’s no moral ambiguity here. There’s only data. And cost. And compassion. Pick two.
And if you’re still scared? Talk to someone who’s been there. Not a rep. Not a website. A real person. I’ll be happy to chat. No judgment. Just facts.
Mohammed Rizvi
January 31, 2026 AT 22:53Bro, I switched to a biosimilar for my psoriasis and now I can afford to take my wife out for dinner once a month instead of just staring at the menu like a hungry ghost. Also, the pen doesn’t hurt as much as the original. Weird, right? 🤫
Curtis Younker
February 1, 2026 AT 11:53OMG I just found out my insurance covers biosimilars now and I’m crying happy tears because I’ve been skipping doses for months because of the cost. Seriously, if you’re on a biologic and haven’t asked about biosimilars, you’re leaving money on the table and risking your health. Go talk to your pharmacist TODAY. They’re not just the people who hand you pills - they’re your secret weapon. I wish I’d known this a year ago. Don’t wait like I did. You deserve to feel better without going broke. 💪❤️
Conor Murphy
February 2, 2026 AT 10:54My cousin in Dublin was on Humira for Crohn’s. Switched to the biosimilar. Same results. Saved €8,000. The only thing different? The pen had a different color cap. She said it felt weird at first - like switching from a Ford to a Toyota. But the engine? Same. She’s now helping other patients navigate the system. Small acts, big ripples.
Uche Okoro
February 3, 2026 AT 17:25The structural heterogeneity of biosimilars introduces stochastic variance in post-translational modifications, which, while statistically insignificant in aggregate, may manifest as clinically relevant immunogenicity in phenotypically sensitive subpopulations. The FDA’s equivalence thresholds are predicated on population-level metrics, not individual pharmacodynamic profiles. This is a systemic epistemic gap.
Aurelie L.
February 4, 2026 AT 03:47I switched. My skin broke out. It was the biosimilar. I know it. I just know it.
Patrick Merrell
February 4, 2026 AT 11:03They’re lying. Biosimilars are just the same drug with a different name so the pharma companies can keep making money. The FDA is in their pocket. I saw a video on YouTube where a guy said the ‘suffix’ is just to track you. They’re putting chips in your blood. I’m not taking it.
bella nash
February 4, 2026 AT 21:49It is imperative that patients engage in a comprehensive, evidence-based dialogue with their prescribing clinicians prior to any therapeutic substitution, particularly when transitioning from reference biologics to biosimilar analogues, given the potential for unforeseen immunological responses and the necessity of maintaining continuity of care within the context of chronic disease management.
Shawn Raja
February 6, 2026 AT 01:31Generics are the people’s drug. Biosimilars? The people’s drug with a PhD. Both are cheaper. Both work. The only thing holding us back is fear - and the people who profit from it. The system wants you scared. Don’t let it win. Ask. Research. Switch. Save. Repeat.
Also, the FDA’s Purple Book? It’s free. Use it. It’s like Google for drugs that don’t want you to know they’re affordable.
Marian Gilan
February 8, 2026 AT 01:14They said biosimilars were safe. But what if the cells they grow them in are secretly controlled by the government? I read a guy on Reddit who said the suffixes are barcodes. They’re tracking who takes what. And the ‘interchangeable’ label? That’s the trigger. One day, they’ll swap your meds without telling you. You’ll be fine… until you’re not. I’m sticking with the brand. $10k? Worth it to stay free.